Candy canes are not only great to eat, but they are also fun to use as part of a winter STEM activity! Your kids will learn what liquid dissolves this holiday sweets the fastest in this dissolving candy canes experiment.

We are having so much fun with candy canes this holiday season! First, we did the bending candy cane experiment, which made us look like we had superpowers and could magically bend candy canes without breaking them. Then we tested our problem-solving skills with the candy cane bridge challenge and helped a bunch of animals cross the bridge.
Now, we are going to answer the question of what dissolves candy cane the fastest. Not only are we going to test if the temperature of the water is a factor (like we did in the dissolving peppermint science experiment), we are also going to see if adding sugar and salt to the water makes candy canes dissolve faster than just pure water.
Dissolving Candy Cane Science Experiment
Materials:
- 6 cups water
- ½ cup sugar, divided
- ½ cup salt, divided
- 6 candy canes
- Timer
- Free dissolving candy cane science experiment worksheet (you can also fill out the form at the end of the post)
Instructions:
- Place 1 cup of water into three different cups. Into one cup, add ¼ cup sugar, stirring until it is dissolved. Into the second cup, add ¼ cup salt, stirring until dissolved.

2. Heat the remaining 3 cups of water until hot.
3. Place 1 cup of hot water into three different cups. Into one cup, add ¼ cup sugar, stirring until it is dissolved. Into the second cup, add ¼ cup salt, stirring until dissolved.
4. Place one unwrapped candy cane into each cup of water. Set a timer for 2 minutes.

5. When the timer goes off, check the candy canes and make note of which have changed.

6. Continue checking the candy canes every 2 to 5 minutes, making note of the time and changes.
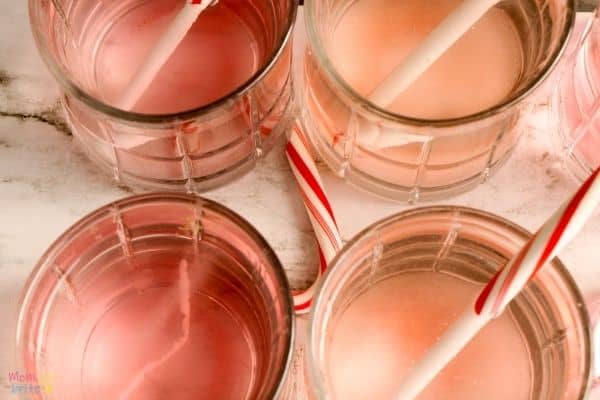
7. Take out the candy canes from the liquids and discuss which liquids caused the candy canes to dissolve faster/slower and why.

Science Behind the Dissolving Candy Cane Experiment
Candy canes are essentially made of sugar, and sugar is made of large molecules called sucrose. These sugar molecules are attracted to each other and held together by the attraction between the polar areas of the sucrose.
However, when you place sugar cane in water, the polar areas of the sugar molecules become attracted to the polar areas of the water molecules. When this attraction is powerful enough to overcome the attraction of the sucrose molecules to each other, the sugar molecules will separate and dissolve.
In this experiment, we also tested whether sugar dissolves faster in hot water versus room temperature water. By the looks of the candy cane in pure hot water, it was obvious that sugar dissolves faster in hot water.

This is because hot water has more energy than room temperature water. In hot water, the water molecules have more energy and move faster, causing them to bump up against the sugar molecules more often.
How about the effect of salt and sugar on how much sugar is dissolved? While salt and sugar are made of different molecules, you can see that they produced similar results when it comes to dissolving the candy cane. In both room temperature water and hot water, the salt and the sugar both seemed to have slowed down the sugar from dissolving.

This is because the salt and sugar molecules in the water are competing with the sucrose from the candy cane for the water molecules. As a result, not as much sugar molecules from the candy cane was able to separate and reattach to the water molecules.
I didn’t get a chance to create a worksheet when we did this experiment, but I made one later! To get your free worksheet where your child can record his observations, just fill out the form below. I also included a blank worksheet in case you want to repeat this experiment with other liquids such as oil, vinegar, and soda!

I would love the form to use with my Kindergarten class!
awesome! simply subscribe and you can get the form delivered to your inbox 🙂